Embryology.
The gut is derived from the endoderm starting at day 16. The embryo folds ventrally and a piece of the yolk sac, an endoderm-lined structure in contact with the ventral aspect of the embryo, begins to be pinched off to become the primitive gut. The yolk sac remains connected to the gut tube via the vitelline duct (the duct usually regresses, if not —-> Meckel's diverticulum).
The primitive gut can be divided into foregut, midgut, and hindgut, each giving rise to specific gut and gut-related structures. Components derived from the gut proper, including the stomach and colon, develop as swellings or dilatations of the primitive gut but gut-related derivatives develop as out-pouchings of the primitive gut. The blood vessels supplying these structures remain constant throughout development.
- The foregut (the anterior part of the alimentary canal, from the mouth to the duodenum at the entrance of the bile duct) becomes the oesophagus to first 2 sections of the duodenum and also gives rise to liver, gallbladder, pancreas, spleen and uperior portion of pancreas
- Supplied by the celiac trunk.
- Pain in the foregut is typically referred to the epigastric region, just below the intersection of the ribs.
- The midgut becomes lower duodenum, jejunum, ileum, cecum, appendix, ascending colon, and first two-thirds of the transverse colon
- Supplied by branches of the superior mesenteric artery.
- Pain in the midgut is referred to the umbilical region.
- The hindgut gives rise to the last third of the transverse colon, descending colon, rectum, and upper part of the anal canal
- Supplied by branches of the inferior mesenteric artery.
- Hindgut pain goes to the suprapubic area.
Anatomy.
Anterior abdominal wall.
Totally muscular anteriorly and laterally. Variable thickness superficial fascia (Camper's) and deep fascia (Scarper's). Intercostal nerves T7-T12 pass forwards between the internal oblique and transversus abdominis to supply both skin and muscles of anterior wall and rectus muscle (T7 skin over xiphoid, T10 umbilicus, T12 suprapubic, L1 divides to give iliohypogastric nerve to skin over pubis and ilioinguinal nerve to anterior part of scrotum and root of penis/labia and clitoris).

There is one vertical muscle and three flat sheets:
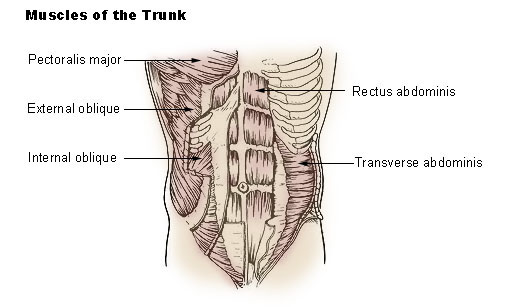
- Rectus abdominis runs vertically both sides divided by linea alba, from xiphisternum & lower costal cartilages to pubic symphysis (it's the sixpack).
- External oblique is the most superficial, runs down and medially from the lower 8 ribs. Pulls the thorax downwards to compress the abdominal cavity. Its inferior margin forms the inguinal ligament.
- Internal oblique runs down and laterally and inferiorly its fibres combine with transversus abdominis fibres to form the cojoint tendon.
- Transversus abdominis supports the trunk, is deep to the internal oblique and runs horizontally.
The peritoneum is a serous membrane that forms the lining of the abdominal cavity (parietal and visceral).
The inguinal ligament runs from the ASIS to the pubic tubercle. The inguinal canal is a passage in the anterior abdominal wall which in men conveys the spermatic cord and in women the round ligament (larger in men). It's just above the medial half of the inguinal ligament and carries ilioinguinal nerve and genital branch of the genitofemoral nerve. About 3.75 cm, oblique directed inferiorly, anteriorly and medially. Visualize the canal as a cylinder, stretching from the deep inguinal ring (2cm above femoral pulse) to the superficial inguinal ring (a deficit in the external oblique). The inguinal ligament forms its base and the conjoint tendon its roof.

The spermatic cords passes through the inguinal canal and carries
- arteries: testicular artery, deferential artery, cremasteric artery
- nerves: nerve to cremaster (genital branch of the genitofemoral nerve), testicular nerves (sympathetic nerves)
- vas deferens (passes posteriorly to the bladder and carries sperm into the prostatic urethra)
- pampiniform plexus
- lymphatic vessels
- tunica vaginalis (remains of the processus vaginalis).
The cremaster covers the testes and raises/lowers them to regulate temperature and promote spermatogenesis. Contraction may also occur during arousal which can prevent injury to the testicles during sex. In a cool environment the cremaster draws the testis closer to the body and reduces surface area thereby reducing heat loss, while when it is warmer the cremaster relaxes allowing the testis to cool by increasing exposed surface area. Contraction can also occur in fight or flight. Clinically, a reflex arc termed the cremasteric reflex can be demonstrated by lightly stroking the skin of the inner thigh downwards from the hip towards the knee. This causes the cremaster muscle on the same side to rapidly contract, raising that testicle. The cremaster can also be contracted voluntarily, by contracting the pubococcygeus muscle, or by sucking in the abdomen.
The femoral canal is located below the inguinal ligament on the lateral aspect of the pubic tubercle. It is bounded by the inguinal ligament anteriorly, pectineal ligament posteriorly, lacunar ligament medially, and the femoral vein laterally. It normally contains a few lymphatics, loose areolar tissue and occasionally a lymph node called Cloquet's node. It is conical in shape, 2cm long and carries efferent lymph vessels and a lymph node. The function of this canal appears to be to allow the femoral vein to expand when necessary to accommodate increased venous return from the leg during periods of activity. The lacunar ligament is the pubic end of the inguinal ligament and its sharp edge can strangulate a femoral hernia; is the only boundary of the femoral canal that can be cut to release a femoral hernia.
Hernias.
Inguinal hernias are of two types:
- Indirect: bulge through the deep inguinal ring into the canal and if big enough, emerge through the superficial ring and look like a scrotal swelling (common in kids), lying lateral to the inferior epigastric artery.
- Direct: caused by a weakness of the conjoint tendon and is common in mature men, bulging through the conjoint tendon between the deep ring and the midline (the inguinal triangle or Hesselbach's triangle), medial to the inferior epigastric artery.
- Congenital inguinal hernias: coils of intestine in the scrotum due to precessus vaginalis not separating from peritonoeal cavity.
Femoral hernias are more common in FEMales and typically present when standing erect as a groin lump or bulge, which may differ in size during the day, based on internal pressure variations of the intestine. The bulge or lump typically is smaller or may not be visible in a prone position. They may or may not be associated with pain. A femoral hernia has often been found to be the cause of unexplained small bowel obstruction. Cough impulse is often absent. The lump is more globular than the pear shaped lump of the inguinal hernia. The bulk of a femoral hernia lies below an imaginary line drawn between the anterior superior iliac spine and the pubic tubercle (which essentially represents the inguinal ligament) whereas an inguinal hernia starts above this line.
A reducible femoral hernia occurs when a femoral hernia can be pushed back into the abdomen, either spontaneously or with manipulation. This is the most common type of femoral hernia and is usually painless. An irreducible femoral hernia occurs when a femoral hernia becomes stuck in the femoral canal. This can cause pain and a feeling of illness.
An obstructed femoral hernia occurs when a part of the intestine becomes intertwined with the hernia, causing an intestinal obstruction. The obstruction may grow and the hernia can become increasingly painful. Vomiting may also result.
A strangulated femoral hernia occurs when a femoral hernia blocks blood supply to part of the bowel - the loop of the bowel loses its blood supply. Strangulation can happen in all hernias, but is more common in femoral and inguinal hernias due to their narrow "necks". Nausea, vomiting, and severe abdominal pain may occur with a strangulated hernia. This is a medical emergency. A strangulated intestine can result in necrosis (tissue death) followed by gangrene (tissue decay).
Posterior abdominal wall.
The psoas major (and in <50% of people, the accompanying psoas minor) arises from the lumbar vertebrae L1-L4 and attaches distally (with iliacus, forming the iliopsoas) to the lesser trochanter of the femur; it's innervated by rami from L1-L3. Contributes to flexion and external rotation in the hip joint. On the lumbar spine, unilateral contraction bends the trunk laterally, while bilateral contraction raises the trunk from its supine position (and so is a hip flexor). When doing a situp that brings the torso (including the lower back) away from the ground and towards the front of the leg, the hip flexors (including the iliopsoas) will flex the spine upon the pelvis. Tightness of the psoas can result in lower back pain by compressing the lumbar discs.
Iliacus arises from the iliac fossa on the interior side of the hip bone, and also from the region of the anterior inferior iliac spine (AIIS), joins the psoas and so is a hip flexor.
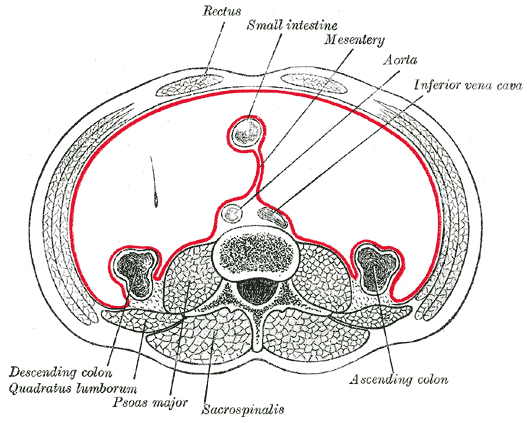
Quadratus lumborum arises from the posterior part of the iliac crest and attaches superiorly to the 12th rib. Can assist inspiration and laterally flexes the column. It is enclosed in thoracolumbar fascia. Anterior to the quadratus lumborum are the colon, the kidney, the psoas major and (if present) psoas minor, and the diaphragm;
The diaphragm is the main muscle of inspiration. The central tendon is fused with the fibrous pericardium. The aortic hiatus at T12 lies behind the diaphragm and carries the thoracic aorta, thoracic duct and azygos vein. The oesophageal opening at T10 is to the left of the midline and carries the esophagus, the anterior and posterior vagal trunks, and some small esophageal arteries. The caval opening at T8 is in the central tendon for the IVC and right phrenic nerve. The IVC walls are fused to the central tendon so when the diaphragm contracts, the IVC dilates and the increased intra-abdominal pressure forces blood into the RA.
The paravertebral gutter (aka pulmonary groove) is the deep vertical recess formed on either side of the thoracic cage by the posterior curvature of the ribs and containing the posterior portions of the lung.
Pelvis: muscles and skeletal framework.
Levator ani is a broad, thin muscle, situated on the side of the pelvis. It is attached to the inner surface of the side of the lesser pelvis, and unites with its fellow of the opposite side to form the greater part of the floor of the pelvic cavity. It supports the viscera in pelvic cavity, and surrounds the various structures which pass through it. In combination with the coccygeus muscle, it forms the pelvic diaphragm. It's pierced by the rectum, urethra and vagina.
Obturator internus arises from the inner surface of the antero-lateral wall of the pelvis, where it surrounds the greater part of the obturator foramen, being attached to the inferior rami of the pubis and ischium, and at the side to the inner surface of the hip bone below and behind the pelvic brim, reaching from the upper part of the greater sciatic foramen above and behind to the obturator foramen below and in front. The tendon inserts on the greater trochanter of the proximal femur. Innervated by L5-S1.

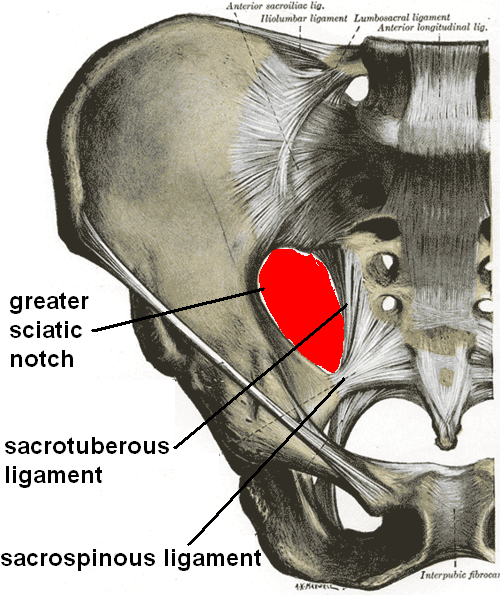
At the anorectal junction, the gut curves backward and its concavity is held by the puborectal sling. The sacrotuberous (lower and back part of the pelvis, running from the sacrum to the tuberosity of the ischium) and sacrospinous ligaments block rotation of the sacrum and ileum around one another. Laxity of these ligaments allows for posterior rotation of the ilium with respect to the sacrum to occur. Stresses occur most often when leaning forward or getting out of a chair.
The pelvis has three bones: the ilium, ischium, and pubis, distinct from each other in the young but fused in the adult; the union of the three parts takes place in and around a large cup-shaped articular cavity, the acetabulum, which is situated near the middle of the outer surface of the bone.
The ilium supports the flank, is the superior broad and expanded portion which extends upward from the acetabulum. The ischium is the lowest and strongest portion of the bone; it proceeds downward from the acetabulum, expands into a large tuberosity, and then, curving forward, forms, with the pubis, a large aperture: the obturator foramen. The pubis extends medially and inferiorly from the acetabulum and articulates in the midsagittal plane at the pubic symphysis, with the bone of the opposite side: it forms the front of the pelvis and supports the external organs of reproduction.
The sacrum is a triangular bone inserted like a wedge between the two hip bones. Its upper part connects with the last lumbar vertebra, and bottom part with the coccyx (tailbone). It consists of usually five initially unfused vertebrae which begin to fuse between ages 16–18 and are usually completely fused into a single bone by age 34. It is oblique forwards and kyphotic, allowing greater room for the pelvic cavity. The coccyx is usually formed of four rudimentary vertebrae (sometimes five or three). It articulates superiorly with the sacrum.
The bladder lies almost entirely within the pelvis and rests on the pubis and pelvic floor. It ascends into the abdomen proper and may reach the level of the umbilicus when full. In infancy even the empty bladder lies mostly within the abdomen proper.
The superior surface and the upper part of the base are covered by peritoneum. As the bladder fills and ascends, the peritoneum is lifted off the abdominal wall; hence the reflection becomes higher. Rupture of the bladder may result in either intraperitoneal or extraperitoneal extravasation of urine. Behind, the peritoneum forms the rectovesical (or uterovesical) pouch. The superior surface is related to the intestine and the body of the uterus. The inferolateral surfaces are related to the retropubic space, which contains veins and a pad of fat. The base faces backward and downward and is related to the seminal vesicles, ductus deferentes, and rectum or to the vagina and supravaginal cervix.
The apex of the bladder is connected to the umbilicus by the median umbilical ligament, which is a remnant of the urachus. The bladder is connected to the umbilicus also by the right and left medial umbilical ligaments, which are the obliterated umbilical arteries. The main part of the bladder is termed its body. The lowest part, or neck, of the bladder is attached to the pelvic diaphragm and is continuous in the male with the prostate. This region is anchored by localized thickenings of the superior fascia of the pelvic diaphragm: the medial and lateral puboprostatic (or pubovesical) ligaments. Another fascial support, the lateral ligament, extends backward on each side from the base of the bladder to the sacrogenital fold.

Lymphatic drainage of the abdomen and pelvis.

The anterior abdominal wall is divided into regions above the umbilicus, which drain to the axillary lymph nodes and below the umbilicus which drain to the superficial inguinal nodes. The mesenteric lymph nodes lie between the layers of the mesentery (the double layer of peritoneum that suspends the jejunum and ileum from the posterior wall of the abdomen). Pre-aortic nodes lie around the three anterior visceral branches of the aorta (coeliac, superior and inferior mesenteric) and drain the territories of these: intestines, liver, gall bladder, spleen, pancreas. Lymph from these nodes drains into the cisterna chyli. The cisterna is a thin-walled lymphatic sac at the lower end of the thoracic duct into which lymph from the intestinal trunk and two lumbar lymphatic trunks flow. It is about 5cm long and lies anterior to the vertebral bodies of L1-L2.
Para-aortic nodes are arranged around the lateral branches of the aorta and drain corresponding territories: kidneys, adrenals and gonads. The common iliac nodes draining the pelvis and lower limbs also drain to these nodes. The lumbar trunks then drain lymph into the cisterna chyli.
The thoracic duct is the main lymphatic channel of the body and receives lymph from everywhere except the right side of the head and neck, the rioght upper limb and right side of the thorax. It begins in the abdomen just below the diaphragm at the cisterna chyli and passes through the aortic hiatus with the aorta and azygos vein. At T4 it crosses to the left and drains in the junction of the left brachiocephalic and jugular veins. Abdominal cancer can spread through it to the left supraclavicular nodes.
The alimentary canal drains into the preaortic lymph nodes. Bowel cancer, especially of the colon, spreads via lymphatics, blood and local peritoneal seeding.
Peritoneal cavity and peritoneum.
The peritoneal cavity is a potential space between the parietal peritoneum and visceral peritoneum, the two membranes that separate the organs in the abdominal cavity from the abdominal wall. It is one of the spaces derived from the coelomic cavity of the embryo, the others being the pleural cavities around the lungs and the pericardial cavity around the heart. The peritoneal cavity is the largest serosal sac in the body and secretes approximately 50 ml of fluid per day. This fluid acts as a lubricant and has anti-inflammatory properties. It is a common injection site, used in intraperitoneal injection. An increase in the capillary pressure in the abdominal viscera can cause fluid to leave the interstital space and enter the peritoneal cavity: ascites.
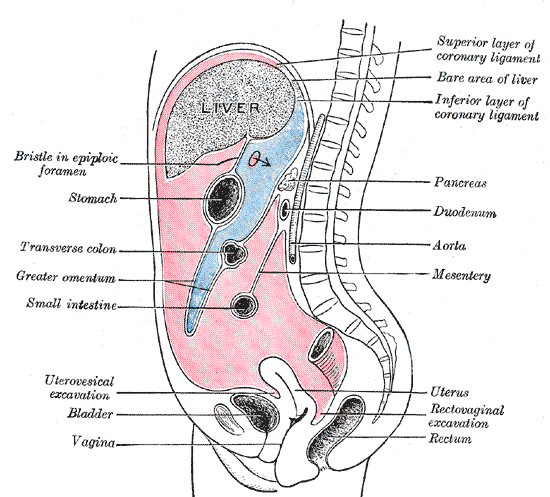
In embryonic development (weeks 4-5), the stomach goes left and the liver enlarges on the right. The space posterior to the stomach is the lesser sac or omental bursa. It is connected to the greater sac by a narrow opening: the epiploic foramen. The pelvic cavity is a body cavity that is bounded by the bones of the pelvis. Its oblique roof is the pelvic inlet (the superior opening of the pelvis). Its lower boundary is the pelvic floor.
The lesser omentum connects the lesser curvature of the stomach and proximal duodenum with the liver. The greater omentum is a double layer that covers the intestines anteriorly like an apron. It contains fat and helps to contain and control inflammation within the peritoneal cavity.
The mesentery of the transverse colon divides the peritoneal cavity into the superacolic compartment (stomach, liver and spleen) and infracolic compartment (small intestine, colon and rectum). Paracolic gutters connect the compartments on either side. The right and left paracolic gutters are peritoneal recesses on the posterior abdominal wall lying alongside the ascending and descending colon. The main paracolic gutter lies lateral to the colon on each side. A less obvious medial paracolic gutter may be formed, especially on the right side, if the colon possesses a short mesentery for part of its length. The right (lateral) paracolic gutter runs from the superolateral aspect of the hepatic flexure of the colon, down the lateral aspect of the ascending colon, and around the caecum. It is continuous with the peritoneum as it descends into the pelvis over the pelvic brim. Superiorly, it is continuous with the peritoneum which lines the hepatorenal pouch and, through the epiploic foramen, the lesser sac. Bile, pus or blood released from viscera anywhere along its length may run along the gutter and collect in sites quite remote from the organ of origin. In supine patients, infected fluid from the right iliac fossa may ascend in the gutter to enter the lesser sac. In patients nursed in a sitting position, fluid from the stomach, duodenum or gallbladder may run down the gutter to collect in the right iliac fossa or pelvis and may mimic acute appendicitis or form a pelvic abscess. The right paracolic gutter is larger than the left, which together with the partial barrier provided by the phrenicocolic ligament, may explain why right subphrenic collections are more common than left subphrenic collections
The hepato-renal pouch or recess is the space that separates the liver from the right kidney. As a potential space, the recess is not filled with fluid under normal conditions but can fill in e.g. ascites.
The small bowel mesentery starts at the duodenojejunal junction just to the left of L2 and extends down to reach the sacroiliac joint at S2. It contains superior mesenteric vessels, lymphatics and autonomic nerves.
The mesoappendix is the portion of the mesentery connecting the ileum to the appendix. It may extend to the tip of the appendix. It encloses the appendicular artery and vein, as well as lymphatic vessels, nerves, and often a lymph node. The transverse mesocolon is a broad, meso-fold of peritoneum, which connects the transverse colon to the posterior wall of the abdomen.
The sigmoid colon (pelvic colon) is the part of the large intestine that is closest to the rectum and anus. It forms a loop that averages about 40 cm. in length, and normally lies within the pelvis, but on account of its freedom of movement it is liable to be displaced into the abdominal cavity. The sigmoid mesocolon is the fold of peritoneum which retains the sigmoid colon in connection with the pelvic wall.

Between the rectum and the bladder, the peritoneal cavity forms, in the male, a pouch, the rectovesical excavation (or rectovesical pouch), the bottom of which is slightly below the level of the upper ends of the vesiculae seminales—i. e., about 7.5 cm. from the orifice of the anus.
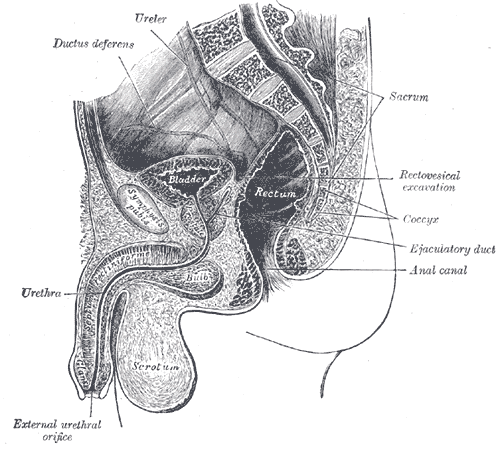
The rectouterine pouch (or rectouterine excavation) is the rectovesical equivalent in the female, an extension of the peritoneal cavity between the rectum and back wall of the uterus. It is used in the treatment of end-stage renal failure in patients who are treated by peritoneal dialysis. The tip of the dialysis catheter is placed into the deepest point of the pouch. In women it is the deepest point of the peritoneal cavity. The pouch on the other side of the uterus is the vesicouterine (or uterovesical) excavation. It is near the posterior fornix of the vagina.

Abdominal reflexes.
Superficial reflexes are motor responses to scraping of the skin. They are graded simply as present or absent, although markedly asymmetrical responses should be considered abnormal as well. These reflexes are quite different from the muscle stretch reflexes in that the sensory signal has to not only reach the spinal cord, but also must ascend the cord to reach the brain. The motor limb then has to descend the spinal cord to reach the motor neurons. As can be seen from the description, this is a polysynaptic reflex. This can be abolished by severe lower motor neuron damage or destruction of the sensory pathways from the skin that is stimulated. However, the utility of superficial reflexes is that they are decreased or abolished by conditions that interrupt the pathways between the brain and spinal cord (such as with spinal cord damage).
Classic examples of superficial reflexes include the abdominal reflex, the cremaster reflex and the normal plantar response. The abdominal reflex includes contraction of abdominal muscles in the quadrant of the abdomen that is stimulated by scraping the skin tangential to or toward the umbilicus. This contraction can often be seen as a brisk motion of the umbilicus toward the quadrant that is stimulated. The cremaster reflex is produced by scratching the skin of the medial thigh, which should produce a brisk and brief elevation of the testis on that side. Both the cremaster reflex and the abdominal reflex can be affected by surgical procedures (in the inguinal region and the abdomen, respectively). The normal planter response occurs when scratching the sole of the foot from the heel along the lateral aspect of the sole and then across the ball of the foot to the base of the great toe. This normally results in flexion of the great toe (a "down-going toe") and, indeed, all of the toes. The evaluation of the plantar response can be complicated by voluntary withdrawal responses to plantar stimulation.
The "anal wink" is a contraction of the external anal sphincter when the skin near the anal opening is scratched. This is often abolished in spinal cord damage (along with other superficial reflexes).
In abdominal reflexes, the afferent pathways are the lower intercostal nerves and the efferent are also the lower intercostal nerves. These anterior divisions of the 7th-11th thoracic intercostal nerves are continued anteriorly from the intercostal spaces into the abdominal wall; hence they are named thoraco-abdominal nerves (or thoracicoabdominal intercostal nerves). They have the same arrangement as the upper ones as far as the anterior ends of the intercostal spaces, where they pass behind the costal cartilages, and between the internal oblique and transversus abdominis to the sheath of the rectus abdominis, which they perforate. They supply the rectus abdominis and end as the anterior cutaneous branches of the abdomen; they supply the skin of the front of the abdomen. The lower intercostal nerves supply the Intercostales and abdominal muscles; the last three send branches to the Serratus posterior inferior. About the middle of their course they give off lateral cutaneous branches.
Most of the pathways that descend the spinal cord have a tonic inhibitory effect on spinal reflexes. For this reason, the net result of lesions that damage the descending tracts is facilitation of reflexes that are mediated at only the level of the spinal cord (a classic example being the muscle stretch reflex). With few exceptions, this means that these spinally mediated reflexes become hyperactive. After acute lesions, spinal reflexes often pass through an initial stage of hypoactivity. This stage has been called "spinal shock" or diaschisis and is more severe and long lasting in proportion to the degree of damage. For example, transection of the spinal cord removes the greatest amount of higher influence and may be associated with weeks of hypoactivity. Small lesions may have little effect on reflexes. When reflexes return after spinal transection, they become extremely hyperactive.
The alimentary canal.
Abdominal oesophagus and stomach
The abdominal oesophagus is 1 cm long. It enters the stoch at an acute angle to the fundus and pierces the diaphragm at T10 to the left of the midline, supported by a muscular sling from the right crus of the diaphragm (accompanied by the anterior and posterior vagal trunks and branches of the left gastric vessels). The stomach lies between the oesophagus and the dudenum across the supracolic compartment, right to left. The stomach fundus is at the 5th rib, with its most superior point in the midclavicular line. The transpyloric plane goes through L1. The stomach-duodenum junction (the **pylorus) is roughly in the midline at L1. The gastroesophageal junction is not actually a proper valve and is sometimes called the cardiac sphincter, cardia or cardias. It prevents backflow of gastric acid and its failure predisposes to cancer in the lower third of the oesophagus.
The stomach is divided into a fundus, body and pyloric region. The pyloric region is divided into two parts:
- the pyloric antrum, which connects to the body of the stomach.
- the pyloric canal, which connects to the duodenum.
The pyloric sphincter (palpable), or valve, is a strong ring of smooth muscle at the end of the pyloric canal which lets food pass from the stomach to the duodenum. It receives sympathetic innervation from the celiac ganglion.

The stomach has a very rich arterial supply from all three branches of the coeliac trunk (coeliac, superior and inferior mesenteric arteries). They form anastomoses on the greater and lesser curvatures (the anterior and posterior surfaces and borders of the stomach).
Anteriorly, the stomach is overlapped by the left lobe of the liver and on the right by the diaphragm and costal margins. A small area is in contact with the peritoneum posterior to the rectus sheath. Posteriorly the lesser sac separates the stomach from the stomach bed (which has the pancreas, mesentery of the transverse colon, spleen, splenic artery, left kidney, suprarenal gland and diaphragm). The greater sac, also known as the general cavity (of the abdomen) or peritoneum of the peritoneal cavity proper, is the cavity in the abdomen that is inside the peritoneum but outside of the lesser sac. The lesser sac is the cavity in the abdomen that is formed by the lesser and greater omentum. The two sacs are connected by the epiploic foramen.
There are two major kinds of hiatus hernia: 95%: the sliding hiatus hernia, where the gastroesophageal junction moves above the diaphragm together with some of the stomach and 5%: rolling (or paraesophageal) hiatus hernia, when a part of the stomach herniates through the esophageal hiatus and lies beside the esophagus, without movement of the gastroesophageal junction.
Hiatal hernia symptoms' resemble many disorders: dull pains in the chest, shortness of breath (caused by the hernia's effect on the diaphragm), heart palpitations (due to irritation of the vagus nerve), and swallowed food "balling up" and causing discomfort in lower esophagus until it passes on to stomach. It is usually symptomless. Risk factors: increased pressure within the abdomen caused by heavy lifting or bending over, frequent or hard coughing, hard sneezing, pregnancy and delivery, violent vomiting, straining with constipation, obesity (extra weight pushes down on the abdomen increasing the pressure), use of the sitting position for defecation, heredity, smoking (why?), drug use e.g. cocaine (why?), stress (why?), diaphragm weakness.
Pyloric stenosis causes severe projectile non-bilious vomiting in the first few months of life. Stenosis of the pyloric sphincter due to hypertrophy of the surrounding muscle causes which spasms when the stomach empties. It is felt classically as an olive-shaped mass in the middle upper part or right upper quadrant of the infant abdomen. It is uncertain whether there is a real congenital narrowing or whether there is a functional hypertrophy of the pyloric sphincter muscle. This condition typically develops in male babies in the first 2-6 weeks of life. Pyloric stenosis also occurs in adults where the cause is usually a narrowed pylorus due to scarring from chronic peptic ulceration.
Duodenum
The small intestine consists of the duodenum, jejunum and ileum. The duodenum is a C-shaped tube about 25 cm long. The first part is free to move and the remainder is retroperitoneal. It has four parts:
- first/superior (5cm) passes posteriorly to the pylorus to the right of the vertebral column and is overlapped by the liver, gallbladder and lies on the bile duct and right kidney (and is susceptible to peptic ulceration), overall roughly along L1
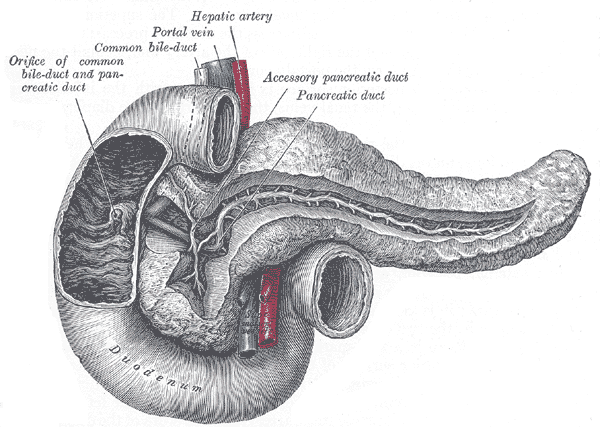
- second/descending part (7.5cm) passes posteriorly and inferiorly curving around the head of the pancreas; the major duodenal papilla, the common opening of the bile and main pancreatic ducts, lies on its posteromedial wall; the transverse colon lies anteriorly
- third/inferior part (10cm) passes to the left, anterior to the IVC and aorta at L3 and posterior to the mensentery
- fourth/ascending part (2.5cm) passes superiorly and anteriorly and is continuous with the jejunum and the duodenojejunal flexure at L2
The sphincter of ampulla or sphincter of Oddi is a muscular valve that controls the flow of digestive juices (bile and pancreatic juice) through the ampulla of Vater (the division between foregut and midgut, where the coeliac artery stops being the blood supply and the superior mesenteric takes over).
The ligament of Treitz connects the duodenum of the small intestines to the diaphragm. It contains a slender band of skeletal muscle from the diaphragm and a fibromuscular band of smooth muscle from the horizontal and ascending parts of the duodenum. When it contracts, the suspensory muscle of the duodenum widens the angle of the duodenojejunal flexure, allowing movement of the intestinal contents.
Small and large intestine, rectum and anal canal
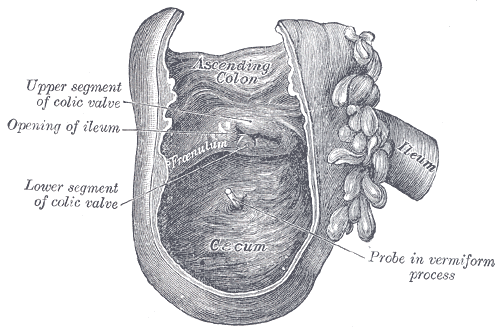
The appendix is located near the junction of the small intestine and the large intestine; its position within the abdomen corresponds to McBurney's point (one-third of the distance from the anterior superior iliac spine to the navel). The base of the appendix is at a fairly constant location, 2 cm below the ileocecal valve; the location of the tip of the appendix can vary from being retrocecal (74%) to being in the pelvis to being extraperitoneal. It averages 11 cm in length (range: 2-20cm). The diameter is usually 7-8mm. The mesoappendix is a mesentery connecting the appendix to the ileum.
The caecum and colon (1.5m long) reabsorb water and propel faeces towards the anus. The external longitudinal muscle coat is concentrated into three bands (teniae coli), which are shorter than the gut tube, gathering up the gut tube into sacculations or haustrations. on the internal surface. The colon has external fatty appendages called appendices epiploicae. The ascending colon lies on the right and is retroperitoneal; at the right colic (hepatic) fixture, it turns transversely across the abodmen, suspended by the transverse mesocolon, allowing it to loop inferiorly. At the left colic (splenic) flexure, the descending colon turns inferirorly and becomes retroperitoneal. The sigmoid colon has a mesentery and meets the rectum in front of S3 (the rectosigmoid junction). The transverse mesocolon is a broad, meso-fold of peritoneum, which connects the transverse colon to the posterior wall of the abdomen.
The jejunum (proximal two-fifths) and ileum (distal three-fifths) of the small intestine are both smooth walled but can be distinguished by the blood supply pattern (jejunum: thicker and more vascular wall, mesentery has clear windows not obscured by fat) and the villi of the jejunum vs Peyer's patches of the ileum. The coils of the jejunum lie in the umbilical region and the ileum occupies the lower abdomen and pelvis. At the junction of the duodenum and jejunum (duodenojejunal flexure), the gut is suspended from the posterior abdominal wall by "the mesentery", which fans out to enclose the superior mesenteric vessels, lymph nodes draining the gut and 6m of small bowel.
The ileocaecal valve, is a papillose structure with physiological sphincter muscle situated at the junction of the small intestine (ileum) and the large intestine, with recent evidence indicating an anatomical sphincter may also be present in humans). Its critical function is to limit the reflux of colonic contents into the ileum. It is the only site in the GI tract which is used for vitamin B12 and bile acid absorption.
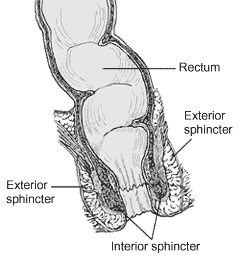
The anal canal is the terminal part of the large intestine, between the rectum and anus below the level of the pelvic diaphragm. It lies in the anal triangle of perineum in between the right and left ischioanal fossa. The anal canal is divided into three parts (zona columnaris is the upper half of the canal, lined by simple columnar epithelium; zona hemorrhagica and zona cutanea, lined by stratified squamous non-keratinized and stratified squamous keratinized, respectively). It is 2.5-4 cm long, extending from the anorectal junction to the anus. It is directed downwards and backwards. It is surrounded by inner involuntary and outer voluntary sphincters which keep the lumen closed in the form of an anteroposterior slit. It is differentiated from the rectum by the transition of the internal surface from endodermal to skinlike ectodermal tissue. Peritoneum covers the front and sides of the upper one-third and the anterior of the middle one-third of the rectum, while the lower one-third is below the level of the peritoneum as it is reflected forwards onto the bladder in men and the uterus in women. The anal canal is completely in the perineum.
The rectal ampulla is the dilated section of the rectum where feces are stored until they are eliminated via the anal canal. The caliber of the rectum at its commencement is similar to that of the sigmoid colon, but near its termination it dilates, forming the ampulla. The rectum intestinum acts as a temporary storage site for feces. As the rectal walls expand due to the materials filling it from within, stretch receptors from the nervous system located in the rectal walls stimulate the desire to defecate (the defaecation reflex), triggering the contraction of rectal muscles, relaxation of the internal anal sphincter and an initial contraction of the skeletal muscle of the external sphincter. The relaxation of the internal anal sphincter causes a signal to be sent to the brain indicating an urge to defecate.
If this urge is not acted upon, the material in the rectum is often returned to the colon by reverse peristalsis where more water is absorbed, thus temporarily reducing pressure and stretching within the rectum. The additional fecal material is stored in the colon until the next mass 'peristaltic' movement of the transverse and descending colon. If defecation is delayed for a prolonged period the fecal matter may harden and autolyze, resulting in constipation.
Once the voluntary signal to defecate is sent back from the brain, the final phase of the cycle begins. The rectum now contracts and shortens in peristaltic waves, thus forcing fecal material out of the rectum and out through the anal canal. The internal and external anal sphincters along with the puborectalis muscle allow the feces to be passed by pulling the anus up over the exiting feces in shortening and contracting actions.
The anal sphincters can be felt by an index finger inserted into the anal canal and rectum.
The following structures are palpable per rectum:
- anteriorly in the male: rectovesical pouch, full bladder, seminal vesicles, displaced or enlarged ductus deferentes, membranous part of urethra when catheterized, and bulbo-urethral glands
- anteriorly in the female: vagina, cervix, ostium uteri, body of uterus when retroverted, recto-uterine fossa, and, pathologically, broad ligaments, uterine tubes, and ovaries
- laterally: ischial tuberosity and spine and sacrotuberous ligament
- Posteriorly: pelvic surface of sacrum and coccyx.
A diverticulum is an outpouching of a structure, e.g. the colon, usually taken to be pathological. In the colon, diverticula are believed to be due to raised intracolonic pressures. Diverticulitis is a digestive disease particularly found in the large intestine, developing from diverticulosis, which involves the formation of pouches (diverticula) on the outside of the colon. Diverticular inflammation causes diverticulitis. Diverticula are susceptible to colonisation by bacteria. Complications include bowel obstruction, peritonitis, bleeding, fistula etc.
Mechanisms of defaecation and anal incontinence.
The anatomy and mechanism of defecation are outlined above under "Small and large intestine, rectum and anal canal". Defecation is normally assisted by taking a deep breath and trying to expel this air against a closed glottis (Valsalva maneuver). This contraction of expiratory chest muscles, diaphragm, abdominal wall muscles, and pelvic diaphragm exert pressure on the digestive tract. The line of division between the internal and external sphincteric muscle is the intersphincteric groove or Hilton's white line. Below it, lymphatic drainage is to the superficial inguinal nodes. Hilton's white line is slightly below the pectinate line. This line represents the transition point from non-keratinized stratified squamous epithelium to simple columnar epithelium in the anus.
The levator ani is a broad, thin muscle, situated on the side of the pelvis, attached to the inner surface of the side of the lesser pelvis, and unites with its fellow of the opposite side to form the greater part of the floor of the pelvic cavity. It supports the viscera in pelvic cavity, and surrounds the various structures which pass through it. In combination with the coccygeus muscle, it forms the pelvic diaphragm. The fibers of the levator ani pass downward and backward to the middle line of the floor of the pelvis; the most posterior are inserted into the side of the last two segments of the coccyx; those placed more anteriorly unite with the muscle of the opposite side, in a median fibrous raphe, the anococcygeal body (or anococcygeal ligament, or anococcygeal raphe), which extends between the coccyx and the margin of the anus.

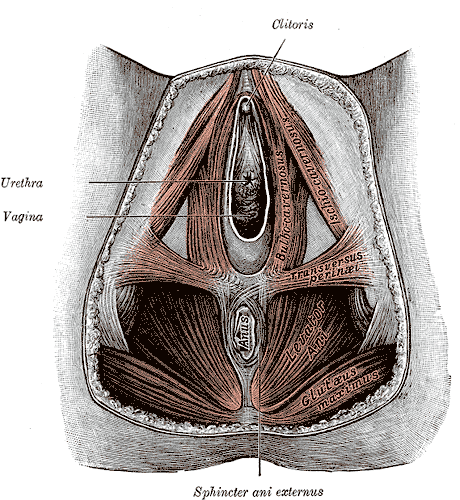
The perineal body (or central tendon of perineum) is a pyramidal fibromuscular mass in the middle line of the perineum at the junction between the urogenital triangle and the anal triangle . It is found in both males and females. In males, it is found between the bulb of penis and the anus; in females, is found between the vagina and anus, and about 1.25 cm in front of the latter. The perineal body is essential for the integrity of the pelvic floor, particularly in females. Its rupture during delivery leads to widening of the gap between the anterior free borders of levator ani muscle of both sides, thus predisposing the woman to prolapse of the uterus, rectum, or even the urinary bladder.
The urogenital triangle is the area bound by a triangle with one vertex at the pubic symphysis and the two other vertices at the ischiopubic rami of the pelvic bone (roughly the top third of the diagram below). The anal triangle - the posterior part of the perineum - has one vertex at the coccyx bone and the others at two ischial tuberosities of the pelvic bone (roughly the bottom of the diagram below). The ischiorectal fossa is somewhat prismatic in shape, with its base directed to the surface of the perineum, and its apex at the line of meeting of the obturator and anal fasciae.
Constipation causes prolonged muscle stretching and leads to weakness of the intestinal muscles. After a certain point, the rectum will no longer close tightly enough to prevent stool loss, resulting in incontinence. Fecal incontinence can be caused by injury to the internal and external anal sphincters (which help retain stool). In women, damage can occur during childbirth. The risk of injury is greatest when the birth attendant uses forceps to help the delivery or does an episiotomy. Hemorrhoid surgery can damage the sphincters as well. A pelvic tumor that grows in or becomes attached to the rectum or anus also can cause muscle damage, as can surgery to remove the tumor.
Blood vessels of the GI tract
The abdominal aorta passes with the azygos vein and thoracic duct through the diaphragm to the left of the midline at T12, lying retroperitoneally on the lumber vertebral bodies and divides into the common iliacs at L4. The paired posterior branches supply the thoracic wall. The subcostal artery lies below the 12th rib and supplies the abdominal wall. Paired lumbar arteries inferiorly supply the posterior abdominal wall and the lumbar regions of the spinal cord. The visceral branches are variable. The renal arteries arise at L1/L2, the adrenal arteries at L1 and the gonadal arteries at L2.
The coeliac trunk or axis supplies the foregut: stomach and first part of duodenum, liver, spleen, most of the pancreas and the omenta. It arises from the anterior aorta 0.5cm below the diaphragm at T12 and rapidly trifurcates into the left gastric, splenic and common hepatic artery. The left gastric rus superiorly in the posterior wall of the lesser sac and divides into oesophageal branches which pass through the oesophageal opening in the diaphragm to supply the lower third of the oesophagus. The left gastric supplies the lesser curvature of the stomach before anastomosing with the right gastric. The splenic artery has a tortuous course in the posterior wall of the lesser sack and passes along the superior border of the pancreas to reach the spleen, giving off short gastric arteries to the fundus of the stomach and the left gastroepiploic artery for the greater curvature of the stomach.
The common hepatic passes inferiorly and to the right in the posterior wall of the lesser sac towards the first part of the duodenum, giving branches to the duodenum (gastroduodenal) and stomach (right gastric). The main trunk, the hepatic artery proper, curves anteriorly to run with the hepatic portal vein and bile duct. Just before entering the porta hepatis, it divides into left and right hepatic.

The superior mesenteric artery supplies the midgut: distal duodenum, uncinate process of the pancreas, small intestine and colon as far as the splenic flexure. It arises at L1 and passes over the left renal vein behind the neck of the pancreas and anterior to the third part of the duodenum, then to the right and from the mesentery of the small intestine, gives off:
- inferior pancreaticoduodenal artery
- iliocaecal (supplies the caecum)
- jejunal and ileal branches (12-15) to supply the small bowel
- right colic, to supply the ascending colon
- middle colic, to supply the proximal two-thirds of the transverse colon
The inferior mesenteric supplies the hindgut: descending colon, sigmoid colon, rectum and the upper part of the anal canal. It arises at L3, passes downward and to the left, crossing the left common iliac and within the mesocolon divides to give: left colic (remainder of the of colon), sigmoid branches (for the most distal parts of the descending and sigmoid) and superior rectal, passing behind the rectum to anastomose with the middle and inferior rectal arteries.
The oesophagus' arterial supply is rich. Branches of the inferior thyroid artery supply the upper esophageal sphincter and cervical esophagus. The paired aortic esophageal arteries or terminal branches of bronchial arteries supply the thoracic esophagus. The left gastric artery and a branch of the left phrenic artery supply the most distal segment. The arteries supplying the esophagus end in an extensive, dense network in the submucosa. The copious blood supply and network of potentially anastomotic vessels may explain the rarity of the esophageal infarction.
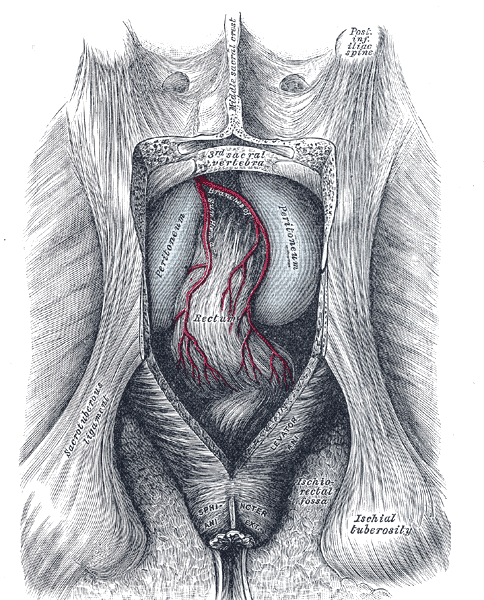
The rectum and anal canal are supplied by the superior rectal artery (the continuation of the inferior mesenteric artery), with assistance from the middle and inferior rectal arteries, and by the median sacral artery.
The IVC lies to the right of the aorta and is formed from the common iliac veins; its main tributaries are renal, adrenal, gonadal and hepatic. It pierces the diaphragm at T8 along with the right phrenic nerve and lies in the posterior wall of the epiploic foramen embedded in the bare area of the liver anterior to the right adrenal. It does not drain the intestine.
The hepatic portal vein carries venous blood from the intestines, spleen and pancreas to the liver for metabolisation. It forms behind the neck of the pancreas from the union of the splenic and superior mesenteric veins. It passes behind the first part of the duodenum lying in front of the IVC and enters through the epiploic foramen. At the porta hepatis, it divides into right and left branches in the same fashion as the hepatic artery and bile duct.
Above the umbilicus, lymphatic drainage is to the axillary nodes while below the umbilicus, drainage is to the superficial inguinal nodes. Lymphatics of the oesophagus, stomach and duodenum drain via the preaortic nodes, which may be surgically inaccessible.
The splanchnic circulation is the circulation of the gastrointestinal tract originating at the coeliac trunk, the inferior mesenteric artery and the superior mesenteric artery. Factors influencing splanchnic blood flow include general hemodyamic factors, the autonomic nervous system, circulating neurohumoral agents, local metabolites and certain properties of splanchnic blood vessels.
General hemodynamic factors include cardiac output, systemic arterial blood pressure, blood volume and the like. Any major decline in these factors, as would occur in hemorrhagic shock, would be reflected by a decline in visceral blood flow.
Both divisions of the autonomic nervous system affect gastrointestinal blood flow. Sympathetic nervous stimuli constrict arteriolar smooth muscle in splanchnic viscera, thereby raising resistance to blood flow through these organs. Sympathetic ischemia seems to be a transient response, however, in the hepatic and mesenteric portions of the splanchnic circulation. Parasympathetic stimulation evokes vasodilation secondary to the increased metabolic activity observed with secretory and motor responses
to cholinergic input.
Circulating neurohumoral agents appear not to be of major consequence as physiological regulators of blood flow to gastrointestinal viscera. However, in severe stress states such as hemorrhagic shock, many vasoconstrictive agents are released
into the blood. These include catecholamines from the adrenal medulla, angiotensin II from the kidneys and vasopressin from the posterior pituitary. These agents reduce blood flow to the viscera.
Anastomoses between systemic venous and hepatic-portal systems take place in the esophagus, umbilicus and anus.
Oesophageal varices) are extremely dilated sub-mucosal veins in the lower third of the esophagus. They are most often a consequence of portal hypertension, commonly due to cirrhosis; patients with esophageal varices have a strong tendency to develop bleeding. Blood from the distal esophagus is drained into the superficial veins lining the esophageal mucosa, which drain into the left gastric vein (coronary vein), which in turn drains directly into the portal vein. These superficial veins (normally only approximately 1mm in diameter) become distended up to 1–2 cm in diameter in association with portal hypertension.
Normal portal pressure is approximately 9 mmHg compared to an inferior vena cava pressure of 2-6 mmHg. This creates a normal pressure gradient of 3-7 mmHg. If the portal pressure rises above 12 mmHg, this gradient rises to 7-10 mmHg. A gradient greater than 5 mmHg is considered portal hypertension. At gradients greater than 10 mmHg, blood flow though the hepatic portal system is redirected from the liver into areas with lower venous pressures. This means that collateral circulation develops in the lower esophagus, abdominal wall, stomach, and rectum. The small blood vessels in these areas become distended, becoming more thin-walled, and appear as varicosities ("Caput medusae").
The superior epigastric artery carries blood from the internal thoracic (or mammary) artery and anastomoses with the inferior epigastric artery at the umbilicus. It supplies the anterior part of the abdominal wall and some of the diaphragm. The superior epigastric arteries, inferior epigastric arteries, internal thoracic arteries and left subclavian artery and right subclavian artery / brachiocephalic are collateral vessels to the thoracic aorta and abdominal aorta. If the abdominal aorta develops a significant stenosis and/or blockage (as may be caused by atherosclerosis), this collateral pathway may develop sufficiently, over time, to supply blood to the lower limbs. A congenitally narrowed aorta, due to coarctation, is often associated with a significant enlargement of the internal thoracic and epigastric arteries.

Haemorrhoids are vascular structures in the anal canal which help with stool control. They become pathological or piles when swollen or inflamed. In their physiological state they act as a cushion composed of arterio-venous channels and connective tissue that aid the passage of stool. The symptoms of pathological hemorrhoids depend on the type present. Internal hemorrhoids usually present with painless rectal bleeding while external hemorrhoids present with pain in the area of the anus. Hemorrhoid cushions are a part of normal human anatomy and only become a pathological disease when they experience abnormal changes. There are three cushions present in the normal anal canal. They are important for continence, contributing to at rest 15–20% of anal closure pressure and act to protect the anal sphincter muscles during the passage of stool.
Predisposing factors are irregular bowel habits (constipation or diarrhea), nutrition (low-fiber diet), increased intra-abdominal pressure (prolonged straining), pregnancy, genetics, absence of valves within the hemorrhoidal veins, aging, obesity and sitting for long periods of time. During pregnancy, pressure from the fetus on the abdomen and hormonal changes cause the hemorrhoidal vessels to enlarge. Delivery also leads to increased intra-abdominal pressures. Complications include bleeding, pain, constipation, infection, stricture, pruritis.
Innervation of alimentary tract.
The subcostal nerve is the ventral ramus of T12 and passes obliquely across quadratus laborum to supply the muscles and skin of the anterior abdominal wall. The lumbar plexus forms from the ventral rami of L1-L4 within the psoas muscle. The resulting nerves (femoral: L2-L4, lateral cutaneous of thigh: L2-L3, ilioinguinal: L1, iliohypogastric: L1, genitofemoral: L1-L2, obturator: L2-L4 and the lumbrosacral trunk) supply various regions of the thigh and hip.
There are complex thoracis plexuses of sympathetic and parasympathetic fibres at the bifurcation of the trachea (pulmonary, cardiac and oesophageal plexuses), with similar arrangements in the abdomen. The preaortic plexuses surround the abdominal aorta and its anterior branches to form the coeliac, superior mesenteric and inferior mesenteric plexuses. Sympathetic fibres synapse in prevertebral ganglia within the plexuses and postganglionic fibres are distributed on blood vessels. Parasympathetic fibres synaps in the walls of the viscera. The superior and inferior hypogastric plexuses are at the bifurcation of the aorta and supply pelvic organs.
The abdominal parasympathetic nerves only supply the gut tube and its derivatives with the anterior and posterior vagal trunks supplying the gut as far as the splenic flexure via the superior mesenteric and coeliac ganglia. Parasympathetic preganglionic fibres are also carried in spinal nerves S2-S4 and form the pelvic splanchic nerves which, via the inferior hypograstric plexus, supply the descending colon, sigmoid colon, rectum and pelvic organs up to the recto-anal junction.
The pudendal (somatic) nerve supplies the anal canal and the external voluntary anal sphincter. Parasympathetic fibers supply the smooth muscle, including the internal sphincter. Sympathetic fibers are mainly vasomotor. Sensory fibers are concerned with the reflex control of the sphincters and with pain. The anal canal is very sensitive below the pectinate line, so that external hemorrhoids may be very painful.
The sympathetic trunks pass behind the medial arcuate ligaments of the diaphragm to enter the abdomen. The main sympathetic supply to the abdominal viscera is from the greater, lesser and least splanchic nerves, which arise from the thoracic sympathetic trunk. These nerves pierce the diaphragm to synapse in the ganglia of the preaortic plexuses. Lumbar splanchic nerves arise from the lumbar region of the sympathetic trunk and synapse in the inferior mesenteric and superior hypogastric plexuses. Postganglionic fibres from the inferior mesenteric plexus supply the distal portions of the colon. There are sympathetic sensory as well as motor fibres. The sensory fibres detect visceral sensation, e.g. distension. The sympathetic chain ends in the pelvis at the ganglion impar.

Generally, parasympathetic stimulation increases gut motility and opens valves while sympathetic stimulation closes valves and inhibits motility. Vagal stimulation in response to smell, taste and sight of food lasts around 30 mins and indirectly causes the parietal cells to secrete HCl and directly stimulates chief cells to secreate pepsinogen to digest proteins.
The enteric nervous system receives parasympathetic (e.g. vagal) and sympathetic (e.g. preverterbral ganglia) but can act independently (e.g. if the vagus nerve is severed, the ENS continues to work). The enteric nervous system is embedded in the lining of the gastrointestinal system. The neurons of the ENS are collected into two types of ganglia: myenteric (Auerbach's) and submucosal (Meissner's) plexuses. Myenteric plexuses are located between the inner and outer layers of the muscularis externa, while submucosal plexuses are located in the submucosa. Through intestinal muscles, the ENS motor neurons control peristalsis and churning of intestinal contents while other neurons control the secretion of enzymes.
Pain in the foregut is typically referred to the epigastric region, just below the intersection of the ribs. Pain in the midgut is referred to the umbilical region. Hindgut pain goes to the suprapubic area.
Cerebrospinal nerves to the parietal peritoneum (T6 through T12) have the same segmental arrangement as the lower thoracic dermatomes. There are no nerve fibers in the visceral peritoneum. Peritoneal pain is steady and is felt over the inflamed area.
Reflexes of the alimentary tract
Vomiting.
Outline: contraction of abdominal muscles and stomach muscles, coupled with relaxation of the cardiac sphincter. Afferent signals are fear, pain, gastric irritation; efferent signals are sympathetic, lower intercostal, phrenic and vagus nerves.
CN X (vagus nerve) is activated when the pharynx is irritated, leading to a gag reflex. The vagal and enteric nervous system inputs transmit information regarding the state of the gastrointestinal system. Irritation of the GI mucosa by chemotherapy, radiation, distention, or acute infectious gastroenteritis activates the 5-HT3 receptors of these inputs. The CNS mediates vomiting that arises from psychiatric disorders and stress from higher brain centers.
The vomiting act has two phases. In the retching phase, the abdominal muscles undergo a few rounds of coordinated contractions together with the diaphragm and the muscles used in respiratory inspiration. In this retching phase nothing has yet been expelled. In the next phase, also termed the expulsive phase, intense pressure is formed in the stomach brought about by enormous shifts in both the diaphragm and the abdomen. These shifts are, in essence, vigorous contractions of these muscles that last for extended periods of time - much longer than a normal period of muscular contraction. The pressure is then suddenly released when the upper esophageal sphincter relaxes resulting in the expulsion of gastric contents. The relief of pressure and the release of endorphins into the bloodstream after the expulsion causes the vomiter to feel better.
Defaecation
Outline: contraction of lower bowel smooth muscle and relaxation of anal sphincters. Afferents: from rectum via pelvic plexus, spinal cord centre; efferents: involvement of parasympathetic, sympathetic and pudendal nerves and abdominal straining.
At the start of the cycle, the rectal ampulla (anatomically also: ampulla recti) acts as a temporary storage facility for the unneeded material. As additional fecal material enters the rectum, the rectal walls expand. A sufficient increase in fecal material in the rectum causes stretch receptors from the nervous system located in the rectal walls to trigger the contraction of rectal muscles, relaxation of the internal anal sphincter and an initial contraction of the skeletal muscle of the external sphincter. The relaxation of the internal anal sphincter causes a signal to be sent to the brain indicating an urge to defecate. The rectum now contracts and shortens in peristaltic waves, thus forcing fecal material out of the rectum and out through the anal canal. The internal and external anal sphincters along with the puborectalis muscle allow the feces to be passed by pulling the anus up over the exiting feces in shortening and contracting actions.
Histology of the digestive tract
There are four morphological layers of the wall: the mucosa, submucosa, mulscularis propria and adventitia. The innermost mucosa is responsible for absorption of digested nutrients and and is richly vascularised and innervated. It has three layers:
- the epithelium, forming the interface between the lumen and tissue
- the lamina propria, a layer of loose connective tissue that transports absorbed material from the mucosa while serving as a first line of immune defence (contains lymphocytes, mostly IgA-secreting B cells) and
- the muscularis propria, made of smooth muscle whose activity keeps the glands and tissue in motion to prevent clogging and to increase food-epitehlium contact.
The inner epithelial wall is arranged in folds called villi, which increase the surface area of the gut and possess a “brush border” of microvilli. These cells express proteins and enzymes for transport and processing of nutrients. The cells forming the epithelia are derived directly from stem cells located in the crypts (the bottoms of the villi) and must be replaced every 2-4 days due to damage from ingested material passing through. Crypt cells are of several additional types
- Paneth cells secrete defensins, proteins that form pores in their membranes
- enteroendocrine cells release hormones
- goblet cells have a LOL name and secrete mucin, which mixes with water to produce mucus for lubrication.
The major functions of enterocytes include:
- ion uptake, including sodium, calcium, magnesium, and iron. This typically occurs through active transport.
- water uptake. This follows the osmotic gradient established by Na+/K+ ATPase on the basolateral surface. This can occur transcellularly or paracellularly.
- sugar uptake. Polysaccharidases and disaccharidases in the glycocalyx break down large sugar molecules, which are then absorbed. Glucose crosses the apical membrane of the enterocyte using the sodium-glucose cotransporter. It moves through the cytosol (cytoplasm) and exits the enterocyte via the basolateral membrane (into the blood capillary) using GLUT2. Galactose uses the same transport system. Fructose, on the other hand, crosses the apical membrane of the enterocyte, using GLUT5. It is thought to cross into the blood capillary using one of the other GLUT transporters.
- peptide and amino acid uptake. Peptidases in the glycocalyx cleave proteins to amino acids or small peptides. Enteropeptidase (also known as enterokinase) is responsible for activating pancreatic trypsinogen into trypsin, which activates other pancreatic zymogens. They are involved in the Krebs and the Cori Cycles and can be synthesized with lipase.
- lipid uptake. Lipids are broken down by pancreatic lipase and bile, and then diffuse into the enterocytes. Smaller lipids are transported into intestinal capillaries, while larger lipids are processed by the Golgi and smooth endoplasmic reticulum into lipoprotein chylomicrons and exocytozed.
- vitamin B12 uptake. Receptors bind to the vitamin B12-gastric intrinsic factor complex and are taken into the cell.
- resorption of unconjugated bile salts. Bile that was released and not used in emulsification of lipids is reabsorbed in the ileum.
- secretion of immunoglobulins. IgA from plasma cells in the mucosa are absorbed through receptor mediated endocytosis on the basolateral surface and released as a receptor-IgA complex into the intestinal lumen. The receptor component confers additional stability to the molecule.
The inner surface of the mucous membrane has depressions/pits (0.12-0.25mm diameter). These are the ducts of the gastric glands with the openings of the gland tubes at the bottom. Gastric glands are simple or branched tubular glands that emerge on the deeper part of the gastric pits, inside the gastric areas and outlined by the folds of the mucosa.
There are three types of glands: cardiac glands (in the proximal part of the stomach), oxyntic glands (the dominating type of gland), and pyloric glands. The cardiac glands mainly contain mucus producing cells. The bottom part of the oxyntic glands is dominated by zymogen (chief) cells that produce pepsinogen (an inactive precursor of the pepsin enzyme). Parietal cells, which secrete hydrochloric acid are scattered in the glands, with most of them in the middle part.
The upper part of the glands consist of mucous neck cells; in this part the dividing cells are seen. The pyloric glands contain mucus-secreting cells. Several types of endocrine cells are found in all regions of the gastric mucosa. In the pyloric glands contain gastrin producing cells (G cells); this hormone stimulates acid production from the parietal cells. ECL (enterochromaffine-like) cells, found in the oxyntic glands release histamine, which also is a powerful stimulant of the acid secretion. The A cells produce glucagon, which mobilizes the hepatic glycogen, and the enterochromaffin cells that produce serotonin, which stimulates the contraction of the smooth muscles.
The surface of the mucous membrane is covered by a single layer of columnar epithelium . This epithelium commences very abruptly at the cardiac orifice, where there is a sudden transition from the stratified epithelium of the esophagus. The epithelial lining of the gland ducts is of the same character and is continuous with the general epithelial lining of the stomach.
The submucosa is made up of collagen and elastin and links the mucosa to the muscular layer of the tract. It contains the large blood vessels and lymphatics that supply the lamina propria and the mucosal nerve plexus also holds the parasympathetic ganglia
of the submucosal plexus. The muscularis propria has an inner circular layer and an outer longitudinal layer, with the myenteric plexus between them. Its synchronous muscular contractions produce peristaltic waves the move food along; peristaltic activity is regulated by the enteric nervous system (see below) and hormones. The adventitia is the outermost layer.
The nerves of the small intestines are derived from the plexuses of parasympathetic nerves around the superior mesenteric artery. From this source, they run to the myenteric plexus (Auerbach's plexus) of nerves and ganglia situated between the circular muscular fibers and the longitudinal muscle fibers of the muscularis externa. From this a secondary plexus, the plexus of the submucosa (Meissner's plexus, submucous plexus, submucosal plexus, plexus submucosus) is derived, and it is formed by branches that have perforated the circular muscular fibers. This plexus lies in the submucous coat of the intestine inside the muscularis propria; it also contains ganglia from which nerve fibers pass to the muscularis mucosae and to the mucous membrane. The nerve bundles of the submucous plexus are finer than those of the myenteric plexus. Its function is to innervate cells in the epithelial layer and the smooth muscle of the muscularis mucosae.
Auerbach's plexus (or myenteric plexus) exists between the longitudinal and circular layers of muscularis externa in the gastrointestinal tract and provides motor innervation to both layers of the mucosa, having both parasympathetic and sympathetic input, whereas Meissner's plexus has only parasympathetic fibers and provides secretomotor innervation to the mucosa nearest the lumen of the gut. It arises from cells in the nucleus ala cinerea, the parasympathetic nucleus of origin for the tenth cranial nerve (vagus), located in the medulla oblongata. The fibers are carried by both the anterior and posterior vagal nerves. It is found in the muscles of the esophagus, stomach, and intestine. The myenteric plexus is the major nerve supply to the gastrointestinal tract and controls GI tract motility.
The stomach is a dilated region of the gut that secretes compounds in both endocrine and exocrine fashion:
- it continues digestion of carbohydrates (following on from the mouth)
- adds acids to food to kill pathogens and promote protein digestion
- produces gastric lipase to aid lipid digestion
- secretes intrinsic factor, which is used in vitamin B12 absorption
- breaks food down into chyme
The fundus of the stomach is the left portion of the stomach's body, and is marked off from the remainder of the body by a plane passing horizontally through the cardiac orifice. As the rounded part of the upper stomach, it allows for an accumulation of stomach gases produced by chemical digestion. It will also store undigested food for up to 1 hour.
The inner lining of the stomach has several mechanisms to resist the effect of gastric juice on itself, but the mucosa of the esophagus does not. The esophagus is normally protected from these acids by a one-way valve mechanism at its junction with the stomach. This one-way valve is called the esophageal sphincter (ES), and this, along with the angle of His formed here, prevents gastric juice from flowing back into the esophagus. During peristalsis, the ES allows the food bolus to pass into the stomach. It prevents chyme, a mixture of bolus, stomach acid, and digestive enzymes, from returning up the esophagus. The ES is aided in the task of keeping the flow of materials in one direction by the diaphragm.
Six main cell types devoted to digestion are found in the stomach: a) simple epithelium lines the gastric mucosa and produces mucus to protect the stomach from low pH, b) parietal or oxyntic cells are found in the upper part of gastric glands and produce acid by dissolving H2CO3 using carbonic anhydrase and also secrete intrinsic factor, c) mucous cells, located between the parietal cells contains granules at the apical surface and have an unclear role, d) chief cells contain pepsinogen-rich granules that in combination with stomach acid get cleaved to pepsin (which degrades digested proteins into peptides and is one of three principal protein-degrading, or proteolytic, enzymes in the digestive system, the other two being chymotrypsin and trypsin), e) enteroendocrine cells, which are at the base of the gastric glands and secrete enzymes and lastly f) step cells, found in the neck of the gastric glands and divide rapidly to replace epithelial cells lost through GI trauma. The replacement cells differentiate as they move up towards the villi.
The pyloric antrum is the location of several important endocrine cells including gastrin-producing G Cells (stimulate acid production) and the luminal-pH-sensitive population of somatostatin producing of D cells (responsible for shutting off acid secretion). The cells are located in gastric pits. There is a second hormone-sensitive population near the fundus.
A lacteal is a lymphatic capillary that absorbs dietary fats in the villi of the small intestine. Triglycerides are emulsified by bile and hydrolyzed by the enzyme lipase, resulting in a mixture of fatty acids and monoglycerides. These then pass from the intestinal lumen into the enterocyte, where they are re-esterified to form triacylglycerol. The triacylglycerol is then combined with phospholipids, cholesterol ester, and apolipoprotein B-48 to form chylomicrons. These chylomicrons then pass into the lacteals, forming a milky substance known as chyle. The lacteals merge to form larger lymphatic vessels that transport the chyle to the thoracic duct where it is emptied into the bloodstream at the subclavian vein.
At this point, the fats are in the bloodstream in the form of chylomicrons. Once in the blood, chylomicrons are subject to delipidation by lipoprotein lipase. Eventually, enough lipid has been lost and additional apolipoproteins gained, that the resulting particle (now referred to as a chylomicron remnant) can be taken up by the liver. From the liver, the fat released from chylomicron remnants can be re-exported to the blood as the triglyceride component of very low density lipoprotein (VLDL). VLDL, also subject to delipidation by vascular lipoprotein lipase, delivers fats to tissues throughout the body and, in particular, the released fatty acids can be stored in adipose cells as triglycerides. As triglycerides are lost from VLDL, the lipoprotein particle becomes smaller and denser (since protein is denser than lipid) and ultimately becomes low density lipoprotein (LDL), which is atherogenic. In contrast to any other route of absorption from the small intestine, the lymphatic system avoids first pass metabolism.
The small intestine is the final site of food digestion and absorbs most substances. It is 6-7 metres long and is typically broken down into the duodenum, jejunum and ileum. The duodenum is a C-shaped region located near the head of the pancreas and
provides the point of entry of the common bile duct into the small intestine near the ampulla of Vater at the major duodenal papilla. The jejunum and ileum are the longest portions of the intestines at about 6 metres. The jejunum starts at the duodenojejunal flexure and and ileum terminates at the ileocaecal junction. There is no clear division between the jejunum and the ileum but the jejunum is slightly wider and more vascularised and its mesentery is less fatty than in the ileum. Peyer's patches, lymphoid nodules containing macrophages, dendritic cells and B and T cells, are found mainly in the lamina propria and
submucosa of the ileum (about 30 in the normal adult).
The small intestine is adapted for nutrient absorption and its surface area is therefore made as large as possible by large folds, villi and microvilli. Crypts of Lieverkuhn are found deep between the villi and produce digestive secretions as well as helping to replace cells lost from the villi and crypts. They contain
- absorptive cells possessing a brush border and enzymes and transporters at the apical surface
- goblet cells, between the absorptive cells, secrete mucus
- Paneth cells deep in the crypts of Lieberkuhn secrete lysozyme defensins to control pathogen growth
- M cells over Peyer's patches, which transport antigens quickly into the patches and
- stem cells at the bottom, which divide at a constant rate, with the daughter cells differentiating as they migrate up the crypt.
The lamina propria of the small intestine projects into the villi, carrying blood vessels, lymphatics, nerve fibres and smooth muscle cells. The muscularis mucosa in the duodenum contains Brunner's glands, which secrete an alkaline fluid that neutralises gastric acid and aids the activity of pancreatic enzymes.
The large intestine or colon is primarily concerned with absorption of water and with excretion. There are no villi. It also absorbs vitamin K (especially important as the daily ingestion of vitamin K is not normally enough to maintain adequate blood coagulation), vitamin B12, thiamine and riboflavin. The longitudinal layer of the muscularis is reduced to 3 strap-like structures known as the taeniae coli. These are shorter than the colon itself and thus produce the large invaginations seen in the large intestine. The wall of the large intestine is lined with simple columnar epithelium. Instead of having the evaginations of the small intestine (villi), the large intestine has invaginations (the intestinal glands). While both the small intestine and the large intestine have mucus-producing goblet cells, they are abundant in the large intestine.
Liver and biliary system
Gross morphology

The liver is located in the right upper quadrant of the abdominal cavity, resting just below the diaphragm. The liver lies to the right of the stomach and overlies the gallbladder. It is connected to two large blood vessels, one called the hepatic artery and one called the portal vein. The hepatic artery carries blood from the aorta, whereas the portal vein carries blood containing digested nutrients from the entire gastrointestinal tract and also from the spleen and pancreas. These blood vessels subdivide into capillaries, which then lead to a lobule. Each lobule is made up of millions of hepatic cells which are the basic metabolic cells.
The falciform ligament is visible on the front (anterior side) of the liver. This divides the liver into a left anatomical lobe, and a right anatomical lobe. Two additional lobes are found
The central area where the common bile duct, hepatic portal vein, and hepatic artery proper enter is the hilum or "porta hepatis". The duct, vein, and artery divide into left and right branches, and the portions of the liver supplied by these branches constitute the functional left and right lobes. The liver gets a dual blood supply from the hepatic portal vein and hepatic arteries. Supplying approximately 75% of the liver's blood supply, the hepatic portal vein carries venous blood drained from the spleen, gastrointestinal tract, and its associated organs. The hepatic arteries supply arterial blood to the liver, accounting for the remainder of its blood flow. Oxygen is provided from both sources; approximately half of the liver's oxygen demand is met by the hepatic portal vein, and half is met by the hepatic arteries. Blood flows through the liver sinusoids and empties into the central vein of each lobule. The central veins coalesce into hepatic veins, which leave the liver. From behind, the lobes are divided up by the ligamentum venosum and ligamentum teres (anything left of these is the left lobe), the transverse fissure (or porta hepatis) divides the caudate from the quadrate lobe, and the right sagittal fossa, which the inferior vena cava runs over, separates these two lobes from the right lobe.
The biliary passages are important surgically because of the frequency of inflammation and of gallstones. The passages are supplied chiefly by the cystic artery (a branch of the right hepatic artery). Pain fibers are carried by the splanchnic nerves, and pain from distension or spasm may be severe. The right and left hepatic ducts emerge from the liver and unite to form the common hepatic duct. This receives the cystic duct from the gallbladder and becomes the common bile duct (or choledochal duct; from Gk, chole, "bile"), which opens into the second part of the duodenum in common with or at least beside the pancreatic duct.
Since the biliary tract is an internal organ, it has no somatic nerve supply, and colicky pain due to infection and inflammation of the biliary tract is not a somatic pain. Rather, pain may be caused by luminal distension, which causes stretching of the wall. This is the same mechanism that causes pain in bowel obstructions.
The path of the biliary tree is as follows: bile canaliculi » canals of Hering » bile ductules (in portal tracts) » intrahepatic bile ducts » left and right hepatic ducts » merge to form » common hepatic duct » exits liver and joins » cystic duct (from gall bladder) » forming » common bile duct » joins with » pancreatic duct » forming » ampulla of Vater » enters duodenum.
The common bile duct runs in the free edge of the lesser omentum, then posterior to the first part of the duodenum and through (or at least enfolded by) the head of the pancreas, ending in the second part of the duodenum. As the bile duct traverses the duodenal wall, its wall acquires the choledochal sphincter (of Oddi) and its lumen becomes narrowed. It is usually united with the pancreatic duct, and the two ducts frequently empty into a common channel (hepatopancreatic ampulla), which enters the duodenum at the greater duodenal papilla.
Blockage of the bile duct by a cancer, gallstones (crystalline concretions formed within the gallbladder by accretion of bile components), or scarring from injury prevents the bile from being transported to the intestine and the active ingredient in bile (bilirubin) instead accumulates in the blood. This condition results in jaundice and pruritis from bile salts depositing in the tissue. In certain types of jaundice, the urine will be noticeably darker, and the stools will be much paler than usual. This is caused by the bilirubin all going to the bloodstream and being filtered into the urine by the kidneys, instead of some being lost in the stools through the ampulla of Vater. Jaundice is commonly caused by conditions such as pancreatic cancer, which causes blockage of the bile duct passing through the cancerous portion of the pancreas; cholangiocarcinoma, cancer of the bile ducts; blockage by a stone in patients with gallstones; and from scarring after injury to the bile duct during gallbladder removal.
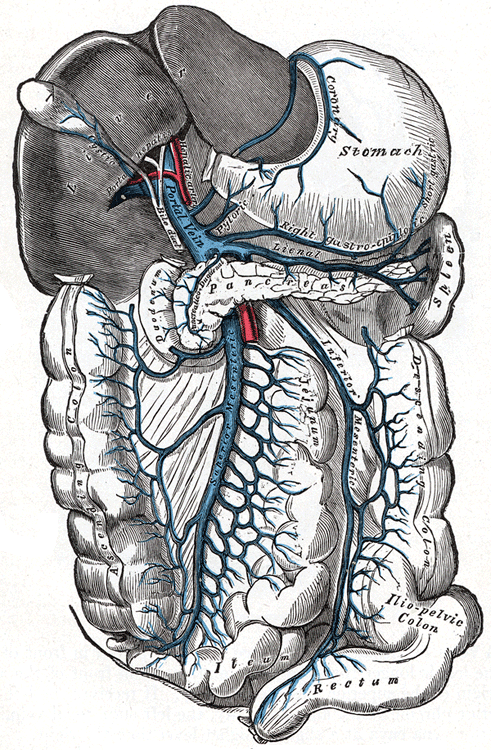
Portal vein hypertension is often defined as a portal pressure gradient (the difference in pressure between the portal vein and the hepatic veins) of 10 mmHg or greater. Causes can be divided into prehepatic, intrahepatic, and posthepatic. Intrahepatic causes include liver cirrhosis, and hepatic fibrosis (e.g. due to Wilson's disease, hemochromatosis, or congenital fibrosis). Prehepatic causes include portal vein thrombosis or congenital atresia. Posthepatic obstruction occurs at any level between liver and right heart, including hepatic vein thrombosis, inferior vena cava thrombosis, inferior vena cava congenital malformation, and constrictive pericarditis.
Consequences of portal hypertension are caused by blood being forced down alternate channels by the increased resistance to flow through the systemic venous system rather than the portal system. They include:
- ascites (free fluid in the peritoneal cavity)
- hepatic encephalopathy.
- increased risk of spontaneous bacterial peritonitis.
- increased risk of hepatorenal syndrome.
- splenomegaly (enlargement of the spleen) with consequent sequestration therein of red blood cells, white blood cells, and platelets, together leading to mild pancytopenia.
- portacaval anastomoses: aesophageal varices, gastric varices, anorectal varices (not to be confused with hemorrhoids), and caput medusae. Esophageal and gastric varices pose an ongoing risk of life-threatening hemorrhage, with hematemesis or melena.
A useful mnemonic is that portal hypertension causes problems in the butt, the gut, and caput.
Symptoms of liver damage:
- pale stools occur when stercobilin, a brown pigment, is absent from the stool. Stercobilin is derived from bilirubin metabolites produced in the liver.
- dark urine occurs when bilirubin mixes with urine
- jaundice (yellow skin and/or whites of the eyes) This is where bilirubin deposits in skin, causing an intense itch. Itching is the most common complaint by people who have liver failure. Often this itch cannot be relieved by drugs.
- oedema of the abdomen, ankles and feet occurs because the liver fails to make albumin.
- fatigue occurs from a generalized loss of nutrients, minerals and vitamins.
- bruising and easy bleeding due to lack of production of clotting factors
Histology of the liver and biliary system
The liver is covered by thin connective tissue (the stroma) which encloses the hepatocytes. The capillary vessels of the liver (sinusoids) have an irregular wall, allowing blood to escape and come into contact with hepatocyte membranes. Hepatocytes group together into lobules, with portal spaces at their corners, where the portal triad can run. A portal triad consists of the following five structures:
- proper hepatic artery
- hepatic portal vein
- bile duct
- lymphatic vessels
- branch of the vagus nerve
A liver sinusoid is a type of sinusoidal blood vessel (with fenestrated, discontinuous endothelium) that serves as a location for the oxygen-rich blood from the hepatic artery and the nutrient-rich blood from the portal vein. Hepatocytes are separated from the sinusoids by the space of Disse. Kupffer cells are located inside the sinusoids and can take up and destroy foreign material such as bacteria (they are an example of the mononuclear lymphocyte system).
Gallstones or bile calculi are formed in the gallbladder, but may pass distally into other parts of the biliary tract such as the cystic duct, common bile duct, pancreatic duct, or the ampulla of Vater. Presence of gallstones in the gallbladder may lead to acute cholecystitis, an inflammatory condition characterized by retention of bile in the gallbladder and often secondary infection by intestinal microorganisms, predominantly Escherichia coli and Bacteroides species. Presence of gallstones in other parts of the biliary tract can cause obstruction of the bile ducts, which can lead to serious conditions such as ascending cholangitis or pancreatitis.
Cholesterol gallstones develop when bile contains too much cholesterol and not enough bile salts. Besides a high concentration of cholesterol, two other factors are important in causing gallstones. The first is how often and how well the gallbladder contracts; incomplete and infrequent emptying of the gallbladder may cause the bile to become overconcentrated and contribute to gallstone formation. The second factor is the presence of proteins in the liver and bile that either promote or inhibit cholesterol crystallization into gallstones. In addition, increased levels of the hormone estrogen as a result of pregnancy, hormone therapy, or the use of combined (estrogen-containing) forms of hormonal contraception, may increase cholesterol levels in bile and also decrease gallbladder movement, resulting in gallstone formation. Gallstone risk factors include overweight, age near or above 40, female, or pre-menopausal; the condition is more prevalent in both North and South American Indians, Hispanics, and those of Northern European descent than other ethnicities. A lack of melatonin could significantly contribute to gallbladder stones, as melatonin both inhibits cholesterol secretion from the gallbladder, enhances the conversion of cholesterol to bile, and is an antioxidant, capable of reducing oxidative stress to the gallbladder.
Pigment stones are small, dark stones made of bilirubin and calcium salts that are found in bile. They contain less than 20% cholesterol.
Symptoms commonly begin to appear once the stones reach a certain size (>8 mm). A characteristic symptom of gallstones is a "gallstone attack", in which a person may experience intense pain in the upper-right side of the abdomen, often accompanied by nausea and vomiting, that steadily increases for approximately 30 minutes to several hours. A patient may also experience referred pain between the shoulder blades or below the right shoulder. These symptoms may resemble those of a "kidney stone attack". Often, attacks occur after a particularly fatty meal and almost always happen at night. Other symptoms include abdominal bloating, intolerance of fatty foods, belching, gas, and indigestion.
Complications are pain (biliary colic), bile duct obstruction (from gallstones being passed down) and infection from stasis in the gallbladder.
Exposure to high levels of bile salts may select for bile-induced-apoptosis-resistant epithelium in the biliary system and the gut, potentially leading to cancer.
Pancreas
Gross morphology
The pancreas develops as ventral and dorsal outgrowths of the duodenum. The ventral pancreatic bud and the adjacent developing liver are carried around the duodenum, bringing the common duct close to the dorsal pancreatic bud. The pancreatic buds fuse; the ventral duct joins with the distal portion of the distal duct to form the main pancreatic duct and the remainder of the dorsal duct forms an accessory pancreatic duct.
It has a head, neck, body, tail and uncinate process ("hook"). The portal vein is formed posterior to the neck of the pancreas by the union of the superior mesenteric and splenic veins. The head and uncinate process lie in the curve and the body and tail pass across the upper posterior abdominal wall to the hilum of the spleen in the transpyloric plane. The pancreas is retroperitoneal, except for the tail. It contains:
- endocrine cells (islets of Langerhans) are of four types and can be classified by their secretion: alpha cells secrete glucagon (increase glucose in blood), β cells secrete insulin (decrease glucose in blood), δ cells secrete somatostatin (regulates/stop alpha and β cells), and PP cells secrete pancreatic polypeptide. These secretions go directly into the bloodstream.
- exocirne cells secrete digestive enzymes, which drain into the duodenum via the pancreatic ducts.
The main duct starts at the tail and joins the accessory duct at the head before joining the bile duct as it pierces the posteromedial wall of the duodenum at the hepatopancreatic ampulla (major duodenal pailla); the accessory duct may open separately into the duodenum at the minor duodenal papilla. The stomach and lesser sac lie anterior to the pancreas. Posterior are the vena cava, aorta, coeliac plexus, left kidney and its adrenal gland and their vessels. The splenic vein crosses behind the adrenal, joining the inferior mesenteric vein and then with the superior mesenteric vein forms the portal vein behind the neck. The splenic artery leas along the superior border of the pancreas. The uncinate process hooks around the superior mesenteric artery. The tail may touch the hilum of the spleen.
The pancreas is supplied by the superior pancreaticoduodenal branch of the coeliac trunk and the inferior pancreaticoduodenal branch of the superior mesenteric artery. The body is supplied by branches of the splenic artery.

Tthe secretory activity of the pancreas is regulated directly via the effect of hormones in the blood on the islets of Langerhans and indirectly through the effect of the autonomic nervous system on the blood flow.
- sympathetic (adrenergic)
- α2: decreases secretion from beta cells, increases secretion from alpha cells
- β2: increases secretion from beta cells
- Parasympathetic (muscarinic)
- M3: increases stimulation of alpha cells and beta cells
The lymphatic drainage is to the adjacent nodes. Pain fibers are carried by the splanchnic nerves.
Cystic fibrosis, also known as mucoviscidosis, is a hereditary disease that affects the entire body, causing progressive disability and early death. It is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The product of this gene helps create sweat, digestive juices, and mucus. The name cystic fibrosis refers to the characteristic 'fibrosis' (tissue scarring) and cyst formation within the pancreas, causing irreversible damage, and often resulting in painful inflammation (pancreatitis).
Compression, obstruction or inflammation of the pancreatic duct may lead to acute pancreatitis. The most common cause for obstruction is choledocholithiasis, or gallstones in the common bile duct. Obstruction can also be due to duodenal inflammation in Crohn's. A gallstone may get lodged in the constricted distal end of the ampulla of Vater, where it blocks the flow of both bile and pancreatic juice into the duodenum. Bile backing up into the pancreatic duct may initiate pancreatitis.
Histology of salivary glands and pancreas
The exocrine tissue of the pancreas is made of acini, spheres of serous cells emptying into a central duct. Between the acini of the pancreas are centroacinar cells which line the ducts. Acinar cells are highly polarised and contain zymogen-rich granules. Control of the exocrine function of the pancreas is via the hormones gastrin, cholecystokinin and secretin, which are hormones secreted by cells in the stomach and duodenum, in response to distension and/or food and which cause secretion of pancreatic juices. Bicarbonate ions, which help neutralise gut acid, are secreted in centroacinar cells, which respond to secretin. Digestive enzymes are produced in basophilic cells, which respond to cholecystokinin.
The precursor enzymes (termed zymogens or proenzymes) are inactive variants of the enzymes; thus autodegradation, which can lead to pancreatitis, is avoided. Once released in the intestine, the enzyme enteropeptidase in the intestinal mucosa activates trypsinogen by cleaving it to form trypsin. The free trypsin then cleaves the rest of the trypsinogen, as well as chymotrypsinogen to its active form chymotrypsin.
Salivary glands are exocrine glands that secrete directly into the mouth. Saliva lubricates the bolus, begins digestion and has some immune functions. Many small glands are found in the mouth in addition to three major gland pairs: the submandibular, lublingual and parotid. Within the glands, two main groups of cells are indentified: mucous and serous. Mucous cells are cuboidal or columnar and are responsible for secretion of mucus. In the submandibular, the tubules are organised in a pattern of mucous cells in tubules capped at the ends with serous cells. Serous cells are pyramidal with a narrow apical membrane decorated with microvilli. They contain many mitochondia, reflecting their roles in the transport of ions. The cells are found in an aciuns (a spherical mass) with a lumen at the centre leading to a duct. The secrete enzymes. Myoepithelial cells or basket cells surround the acini of serous cells and possess muscular elements to allow contractions that expels the glandular secretions.
The secretory portions of the gland empty into intercalated ducts line by cuboidal epithelium. Intercalated ducts join to form interlobular ducts that converge in striated and then excretory ducts. The large salivary glands are encased in connective tissue that divides them into loves. Parasympathetic stimulation directly stimulates secretion (vagus nerve). There is no direct sympathetic stimulation but indirectly, sympathetically-modulated blood flow to the glands has an effect.
Gastrointestinal physiology
Gastrointestinal motility
The GI tract generates motility using smooth muscle subunits linked by gap junctions. These subunits fire spontaneously in either a tonic or a phasic fashion. Tonic contractions are those contractions that are maintained from several minutes up to hours at a time. These occur in the sphincters of the tract, as well as in the anterior stomach. Phasic contractions, consist of brief periods of both relaxation and contraction, occurring in the posterior stomach and the small intestine, and are carried out by the muscularis externa.
Stimulation for these contractions likely originates in modified smooth muscle cells called interstitial cells of Cajal. These cells cause spontaneous cycles of slow wave potentials that can cause action potentials in smooth muscle cells. They are associated with the contractile smooth muscle via gap junctions. These slow wave potentials must reach a threshold level for the action potential to occur, whereupon Ca2+ channels on the smooth muscle open and an action potential occurs. As the contraction is graded based upon how much Ca2+ enters the cell, the longer the duration of slow wave, the more action potentials occur. This in turn results in greater contraction force from the smooth muscle. Both amplitude and duration of the slow waves can be modified based upon the presence of neurotransmitters, hormones or other paracrine signaling. The number of slow wave potentials per minute varies based upon the location in the digestive tract. This number ranges from 3 waves/min in the stomach to 12 waves/min in the intestines.
The patterns of GI contraction as a whole can be divided into two distinct patterns, peristalsis and segmentation. Occurring between meals, the migrating motor complex is a series of peristaltic wave’s cycles in distinct phases starting with relaxation followed by an increasing level of activity to a peak level of peristaltic activity lasting for 5–15 minutes. This cycle repeats every 1.5–2 hours but is interrupted by food ingestion. The role of this process is likely to clean excess bacteria and food from the digestive system.
Peristalsis is one of the patterns that occur during and shortly after a meal. The contractions occur in wave patterns traveling down short lengths of the GI tract from one section to the next. The contractions occur directly behind the bolus of food that is in the system, forcing it toward the anus into the next relaxed section of smooth muscle. This relaxed section then contracts, generating smooth forward movement of the bolus at between 2–25 cm per second. This contraction pattern depends upon hormones, paracrine signals, and the autonomic nervous system for proper regulation.
Segmentation also occurs during and shortly after a meal within short lengths in segmented or random patterns along the intestine. This process is carried out by longitudinal muscles relaxing while circular muscles contract at alternating sections thereby mixing the food. This mixing allows food and digestive enzymes to maintain a uniform composition, as well as to ensure contact with the epithelium for proper absorption.
Swallowing happens in a series of steps. The tongue pushes the food up against the roof of the mouth, forming a bolus. The muscles of the pharynx and tongue voluntarily guide the food into the oesophagus using constriction of pharyngeal muscles. The epiglottis is closed, preventing food from entering the trachea. Lastly, a wave of constriction behind the bolus pushes it into the oesophagus.
Mixing occurs mainly in the pylorus of the stomach. Small amounts of food are forced into the small intestine through the pyloric sphincter. The fat and acid contents of the stomach can be sensed along with the stage of breakdown of food. Local hormone secretion modulates the rate of gastric activity while chemoreceptors signal to the CNS to modulate activity at the system level via parasympathetic nerves.
In addition to segmentation, pendular motion can be induced in individual gut compartments, whereby food is moved back and forth vigorously to mix contents. Movement of food from the small to large intestine occurs through the ileocaecal valve. Sensors within the gut detect stretch, chemical stimuli or irritators. Intrinsic smooth muscle activity results from large migrating motor complexes - large waves of electrical activity accompanied by muscular contractions that migrate down the small intestine during fasting.
Large intestinal motility is driven by peristalsis stimulated by distension sensed and acted upon by the enteric nervous system.
When food containing fat enters the digestive tract, it stimulates the secretion of cholecystokinin (CCK). In response to CCK, the adult human gallbladder, which stores about 50 mL of bile, releases its contents into the duodenum. The bile, originally produced in the liver, emulsifies fats in partly digested food. During storage in the gallbladder, bile becomes more concentrated which increases its potency and intensifies its effect on fats. Cholecystokinin is a hunger suppressant as well as playing a role in drug tolerance to opioids like morphine and heroin; it is partly implicated in experiences of pain hypersensitivity during opioid withdrawal.
Laxatives improve gut motility. Lack of motility can result from inadequate hydration or decreased bowel movement. Hydration and fibre-rich diets can therefore help. Lubricants, e.g. liquid paraffin, help faecal movement. Bulk formers stimulate gut activity and decrease transit time, which help with water retention and therefore lubrication. Fibre and Sterculia are used clinically. Osmotic laxatives act by promoting water retention and are rarely used because the dose is difficult to control and diarrhoea is a frequent result. Motility stimulants promote peristaltic activity through stimulation of the enteric nervous system. They promote very strong contractions and are not used after surgery. Bisacodil is used clinically.
Treatment for diarrhoea often focuses on correction of water loss and ion balance, sometimes accompanied by glucose or starch, aiding absorption. If it is a result of infection, treatments reducing gut motility can prolong infection risk. Kaolin, an adsorbing agent, can be used.
Antiemetics prevent vomiting by antagonising receptors involved in triggering vomiting. H1- and H3- receptor histamine antagonists and muscarinic antagonists are effective. Domperidone can be used with chemotherapy drugs to reduce their effects. Prochlorperazine is a dopamine receptor antagonist that counteracts the vomit-inducing effects of morphine administered for pain relief.
Opiates often inhibit colonic motility and cause constipation. Parasympathomimetics stimulate parasympathetic activity and therefore motility. Exercise and fibre-rich diets enhance motility.
Defecation is under both voluntary and autonomic control. Reflex pathways increase abdominal pressure and allow sphincter relaxation while the pelvic floor is under somatic control. The inner anal sphincter has circular and longitudinal smooth muscle and is under autonomic control; the outer layer has striated muscle controlled both voluntarily and autonomically. During voiding, adrenergic nerves release noradrenaline, which acts on alpha1-adrenoceptors found on muscle cells in both layers, to trigger contraction. Peristaltic activity in the colon triggers the movement of the colon contents into the rectum. Sensory nerve fibres in the gut wall provide the CNS and enteric nervous system with information about rectal state, which can be used to trigger the rectosphincteric reflex, inhibiting the anal sphincters and triggering reflex peristalsis in the rectum to expel the contents. The sphincters can be voluntarily contracted to prevent voiding.
Gastrointestinal secretion
Most digestive enzymes empty into the GI tract through the ducts at the ampulla of Vater that links the gut to the gallbladder, pancreas and liver. The secretory apparatus of the GI tract recycles the secretions and fluid so that the total volume of fluid secreted into and reabsorbed from the gut every day exceeds that ingested.
The salivary glands secrete around 1 L of fluid per day, 80-90% of which is produced by the parotid, submandibular and sublingual glands. Saliva contains ions, mucins and enzymes. Its roles are to moisten the mucosa, initiate digestion through salivary amylase, lubricate the upper GI tract for eating and speech and protect from pathogens. Secretion is from serous cells and is initially isotonic. Chlorine and bicarbonate are secreted by a symporter, as is potassium. Sodium movement is passive, following the anions. Saliva is typically alkaline. At low flow rates, chlorine and sodium are reabsorbed and potassium is reabsorbed, generating a hypotonic solution. At high flow rates, the secretions resemble plasma because there is less time for the transporters to remove ions. Still, saliva is usually hypotonic and contains more potassium than plasma.
Alpha amylase breaks down starch and salivary amylase initiates fat digestion. Mucins are antimicrobial and lubricate the bolus. Proline-rich proteins are antimicrobial and lubricants as well as binding calcium and thus contributing to the strength of tooth enamel. Secretion is regulated in a complex manner and is mainly under parasympathetic control but sympathetic stimulation can act indirectly by reducing blood flow to the glands. The CNS nuclei regulating salivary activity are at the junction of the pons and medulla.
Gastric juice has four groups of components:
- stomach acid, secreted to kill pathogens and aid the other secretions
- mucus, secreted to protect the mucosa from the acid
- pepsin and other enzymes to digest food
- intrinsic factor protects vitamin B12 from acid degradation and is necessary for its absorption
Intrinsic factor (IF),also known as gastric intrinsic factor (GIF), is a glycoprotein produced by the parietal cells of the stomach. It is necessary for the absorption of vitamin B12 in the small intestine. B12 binds to intrinsic factor and the complex travels to the ileum, where special epithelial cells endocytose it. Inside the cell, B12 dissociates and is absorbed. Pernicious anaemia is an autoimmune disorder usually caused by antibodies against intrinsic factor. Erythrocytes are the first to be affected due to their large turnover (because B12 is essential for cell division). Treatment is with regular injections of vitamin B12.
Pancreatic secretions contain many enzymes secreted in inactive form as proenzymes: trypsinogen, chymotripsnogen, procarboxypeptidase etc. These are activated through the action of enterokinase in the intestinal mucosa. Trypsin, chymotrypsin and other pancreatic enzymes cleave proteins into small fragments, accounting for 50% of protein digestion.
Biliary secretions serve three functions:
- emulsify fat in ingested food to make it able to be absorbed by the gut
- neutralise stomach acid by establishing a mildly alkaline environment in which pancreatic enzymes function optimally
- as a vehicle for the excretion of bilirubin and other substances not excreted by the kidneys
Bile contains:
- bile acids, to emulsify lipids, increasing the surface area on which lipolytic enzymes can act. At a sufficiently high concentration, bile acids induce the formation of lipid micelles
- lecithin increases the amount of cholesterol that can be solubilised within micelles
- bile pigments are excreted products of haem metabolism
Bile salts and acids are synthesised by hepatocytes. The gallbladder stores bile and concentrates it for release during digestion. The Na+ / K+ ATPase on the basolateral membrane of gallbladder epithelium draws water out of the lumen by transcellular and paracellular routes; the net action of the transporters result in the progressive addition of H+ into bile, neutralising the HCO3- already present. Only a small proportion of bile salts is excreted; most are reabsorbed in the terminal ileum and returned to the liver where they are re-secreted by hepatocytes. The total bile acid pool is small and may be secreted several times during a meal. 20% of bile acid is lost per day and must be replenished.
Gastric acid secretion and peptic ulceration
Secretion of gastric acid is due to the activity of the H+/K+ ATPase which transports K+ into parietal cells in return for H+ secretion. The potassium can leave the parietal cells through potassium channels, allowing it to re-enter the stomach. Stomach pH is around 2.5, a potentially dangerously low level; hydrogen ion secretion is therefore tightly regulated:
- paracrine regulation is achieved by the enterochromaffin-like (ECL) cells, which secrete histamine; this triggers H+ secretion through its action on H2 receptors on parietal cells.
- parasympathetic nerves derived from the vagus can release ACh, which acts directly on M3-receptors on parietal cells to stimulate H+ release. ACh also acts on the enterochromaffin-like cells to stimulate histamine release
- Gastrin is released by GI tract cells and acts through the cholecystokinin B (CCK-B) receptor on both parietal and ECL cells to stimulate histamine release.
Increased acid secretion is accomplished through a second messenger cascade within parietal cells, which in turns results in the insertion of additional H+/K+ ATPase proteins into the cell membrane.
The chief cells of the stomach release pepsins into the gut. Pepsin is stored in the cells as inactive zymogens (pepsinogen). The low pH of the gut triggers cleavage of the inactive pepsinogen into the active pepsin form, which is irreversibly inactivated in the small intestine by the neutral pH there. The action of pepsin in the gut accounts for around 20% of protein digestion.
An ulcer is an area of epithelial damage. In the stomach this is the result of acid eroding away the mucosa; peptic ulcer can lead to bleeding. The continual damage and repair of mucosa can promote neoplasms. Predisposing factors for gastric ulcers are smoking, alcohol, hypoglycaemia, excess acid (hypoglycaemia or gastrin-secreting tumour) and loss of mucosal protection due to defects in mucus productions. H pylori infection is a causative factor, with elements of the bacterium mimicking molecules expressed by the gastric epithelium, triggering autoimmune attack. Stress is a predisposing factor to H pylori infections.
Peptic ulcers can be treated with direct antacids (e.g. aluminium hydroxide), histamine antagonists (e.g. ranitidine) and blockers of the H+/K+ ATPase (e.g. omeprazole) - sometimes called proton pump inhibitors. Antibiotics against H pylori (clarithromycin and amoxicillin) are effective but resistance is emerging.
Activity of alimentary tract following a meal
The nervous system and endocrine system collaborate to increase gastric secretion and motility when food is eaten and to suppress them as the stomach empties. Gastric activity is divided into the cephalic phase, gastric phase, and intestinal phase, based on whether the stomach is being controlled by the brain, by itself or by the small intestine, respectively. These phases overlap and all three can occur simultaneously.
The cephalic phase is the stage in which the stomach responds to the sight, smell, taste, or thought of food. These sensory and mental inputs converge on the hypothalamus, which relays signals to the medulla oblongata. Vagus nerve fibers from the medulla stimulate the enteric nervous system of the stomach which, in turn, stimulates gastric secretion via ACh release and its actions on M3-receptors on parietal cells to stimulate H+ release. ACh also acts on the enterochromaffin-like cells to stimulate histamine release.
The gastric phase is a period in which swallowed food and semidigested protein (peptides and amino acids) activate gastric activity. About two-thirds of gastric secretion occurs during this phase. Ingested food stimulates gastric activity in two ways: by stretching the stomach and by raising the pH of its contents. Stretch activates two reflexes: a short reflex mediated through the myenteric nerve plexus, and a long reflex mediated through the vagus nerves and brainstem.
Gastric secretion is stimulated chiefly by three chemicals: acetylcholine (ACh), histamine and gastrin. ACh is secreted by parasympathetic nerve fibers of both the short and long reflex pathways. Histamine is a paracrine secretion from the enteroendocrine cells (enterochromaffin-like cells) in the gastric glands. Gastrin is a hormone produced by enteroendocrine G cells in the pyloric glands. All three of these stimulate parietal cells to secrete hydrochloric acid and intrinsic factor. The chief cells secrete pepsinogen in response to gastrin and especially ACh (ACh also stimulates mucus secretion).
As dietary protein is digested, it breaks down into smaller peptides and amino acids, which directly stimulate G cells to secrete more gastrin – a positive feedback loop that accelerates protein digestion. Small peptides also buffer stomach acid so that the pH does not fall excessively. As digestion continues and these peptides are emptied from the stomach, pH drops below 2, at which point stomach acid inhibits the parietal cells and G cells- a negative feedback loop that winds down the gastric phase as the need for pepsin and H+ declines.
The intestinal phase is a stage in which the duodenum responds to arriving chyme and moderates gastric activity through hormones and nervous reflexes. The duodenum initially enhances gastric secretion, then inhibits it. Stretching of the duodenum accentuates vagal reflexes that stimulate the stomach; peptides and amino acids in the chyme stimulate G cells of the duodenum to secrete more gastrin, which further stimulates the stomach. The accumulating acid and semi-digested fats in the duodenum trigger the enterogastric reflex – the duodenum sends inhibitory signals to the stomach by way of the enteric nervous system and sends signals to the medulla that (1) inhibit the vagal nuclei, thus reducing vagal stimulation of the stomach and (2) stimulate sympathetic neurons, which send inhibitory signals to the stomach. Chyme also stimulates duodenal enteroendocrine cells to release secretin and cholecystokinin. These primarily stimulate the pancreas and gall bladder but also suppress gastric secretion and motility. The result is a decline in gastrin secretion and the contraction of the pyloric sphincter to limit the admission of more chyme. The enteroendocrine cells also secrete glucose dependent insulinotropic peptide, which stimulates insulin secretion in preparation for processing the nutrients about to be absorbed by the small intestine.





